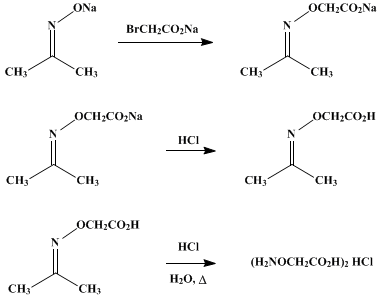
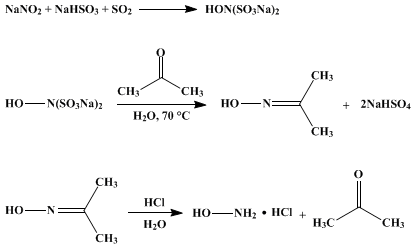
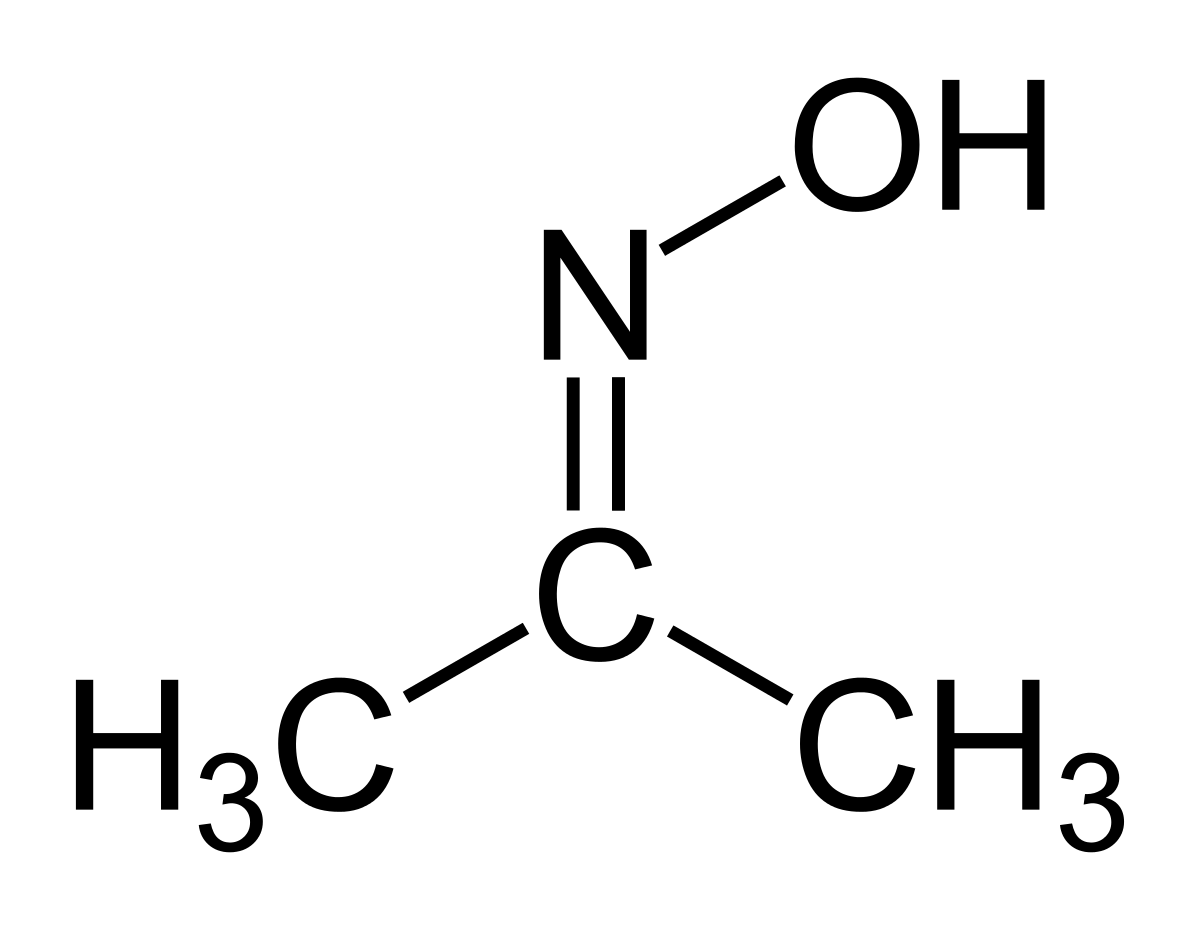
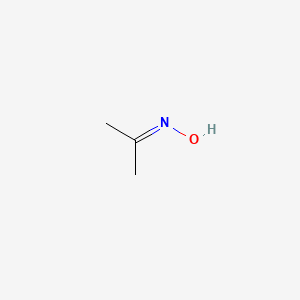
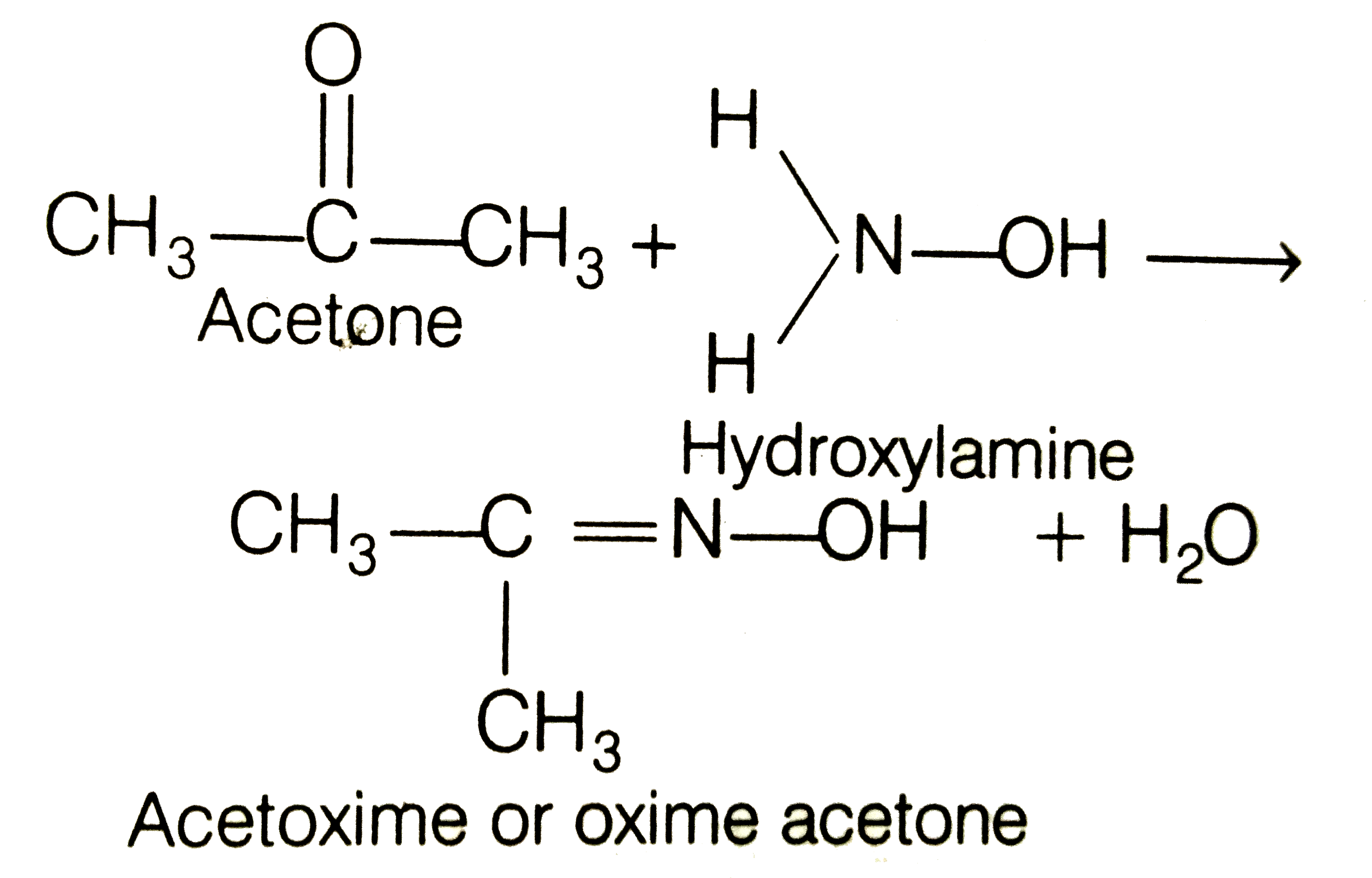
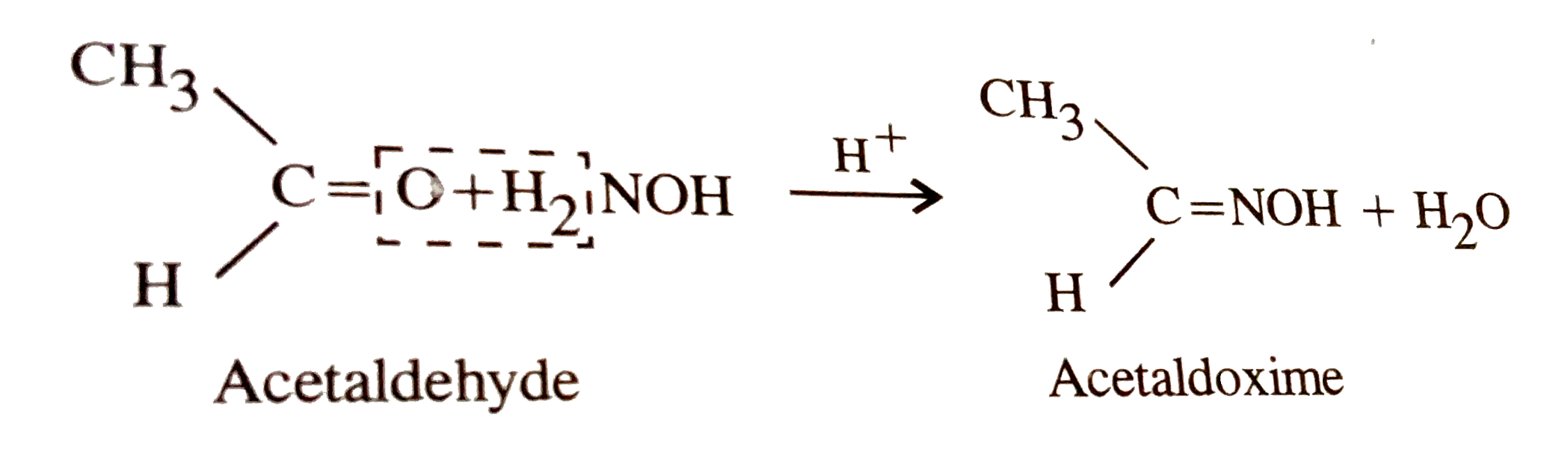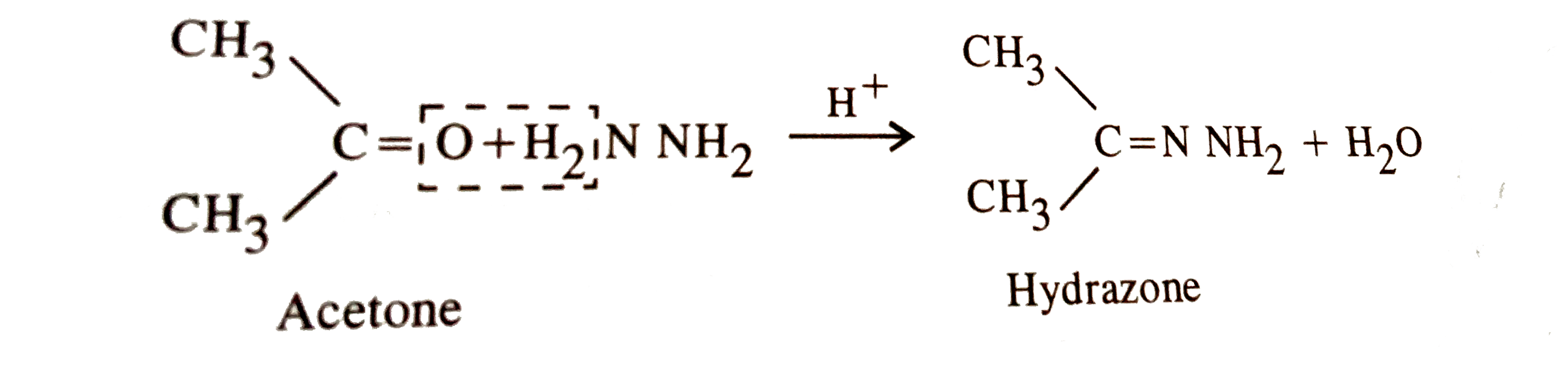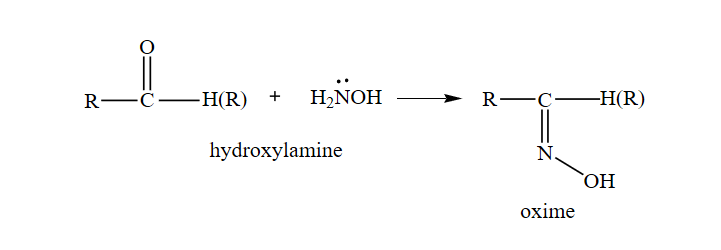What is the action of hydroxyl amine (NH2OH) on : (1) acetaldehyde (2) acetone? - Sarthaks eConnect | Largest Online Education Community
What is the action of hydroxyl amine (NH2OH) on : (1) acetaldehyde (2) acetone? - Sarthaks eConnect | Largest Online Education Community

Acetone is treated with hydroxylamine and the derivative so formed is subjected to catalytic hydrogenation. Final product is

Give one reason each for the following : (i) Acetone reacts with hydroxylamine to form only one product which has no geometrical isomer, but acetaldehyde reacts with hydroxylamine to form a product
The spectra of the CH3COCHO/N2 (a), NH2OH/N2 (b) and CH3COCHO/NH2OH/N2... | Download Scientific Diagram

Molecules | Free Full-Text | New Trends in Diaziridine Formation and Transformation (a Review) | HTML
Unit Operations Laboratory Liquid-Liquid Extraction Titration mechanism 1. Reaction between NH2OH and Acetone NH2OH·HCl + C3H6

Reagents: a NH2OH x HCl; NaHCO3, EtOH; b Na/PrOH; c ClCOOEt, TEA, DMF,... | Download Scientific Diagram
![OneClass: Predict the major product(s) from the treatment of acetone with the following: [H^+], NH_3,... OneClass: Predict the major product(s) from the treatment of acetone with the following: [H^+], NH_3,...](https://prealliance-textbook-qa.oneclass.com/qa_images/homework_help/question/qa_images/109/10906910.jpeg)
OneClass: Predict the major product(s) from the treatment of acetone with the following: [H^+], NH_3,...